Scaffolds and 3D electrodes
Compartmentalized microfluidic-ME-hybrid system
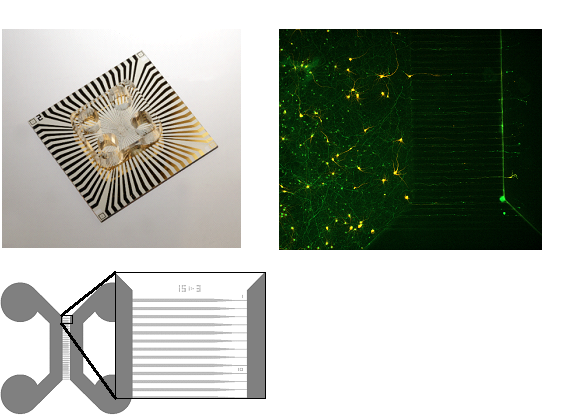
The study of specific neuronal networks is of big importance for understanding the development and progress of various neurodegenerative diseases. Most of the research is based on in vivo approaches, while the reliable in vitro models are still lacking. In this direction of research, we are developing a microfluidic-MEA-hybrid system, which enables the mimicking of specific neuronal pathways from the brain. To this end, we developed a MEA (micro-electrode array) transparent chip with a polyimide surface, which can be reversibly connected with PDMS-based cell culture systems. In this system, different co-cultures are connected via asymmetric funnel-shaped microchannels, enabling an isolated and unidirectional growth of axons within the microchannels. This allows to construct a unidirectional, highly oriented cortico-striatal network with a spatial separation of soma and axons. In combination with the MEA, the system gives the opportunity to measure electrophysiological activity of connected neural network populations and follow the propagation of action potentials along the axons. With the goal of improving the unidirectionality of the microchannels, various designs are being proposed and tested by defining the axonal presence within the microchannels with immunostaining techniques.
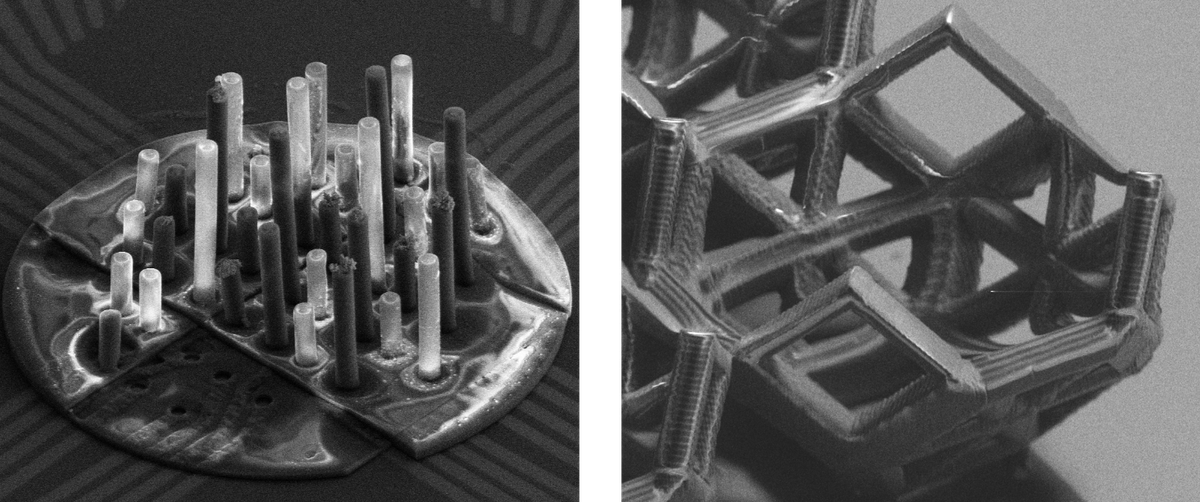
3D cultures
In vitro models of the human brain have started to deploy their potential for better understanding neurological disorders such as Alzheimer's and Parkinson's diseases. Micro-structured devices can help mimic the organization of neural tissue at single cell level which opens new routes to physiologically relevant in vitro models. In contrast to 2D cultures, 3D cultures provide a more complex environment with longer lifespan and also being more informative and predictive, which leads to a better complement to animal models. For 3D cultures scaffolds as growth supporting material are needed. One can distinguish between two families of methods. Either soft or hard biomaterials can be used. Soft biomaterials have the advantage that cells can grow with high degree of freedom and mimic the soft environment of biological tissues. Nevertheless these methods generates random, not spatially controllable 3D neuronal networks. Mostly hydrogels are used as supporting material for soft scaffolds. In contrast using hard biomaterials the growth is supported by solid scaffolds made out of PDMS, graphene, photoresist scaffolds and many more. Solid scaffolds enable higher structural complexity and increased cell survival. Being able to control the cell growth via the scaffold design which results in a spatially well defined cell growth is another huge advantage.

Direct laser writing (DLW) by multi-photon polymerization (MPP) has recently been demonstrated as an attractive method for fabricating precisely controlled micro size cell scaffolds for 3D neuronal networks. It is a three-dimensional printing technology, based on the photon absorption by photopolymers. Due to the non-linearity physics of the absorption process the fabrication of polymer structures with sub-100 nm resolution has been shown. In our group the two-photon absorption (TPA) of photosensitive materials is used to create well controlled solid 3D scaffolds in the µm range. Using the TPA process it is possible to print structures with high complexity while maintaining a high resolution. TPA is defined as the simultaneous absorption of two photons of identical or different wavelengths in order to excite an electron of the photon initiator molecule from the ground to the excited state. This usually triggers the molecule to polymerize.
With further integrating 3D electrodes into the printed 3D scaffolds we are able to record electrical signals from the spatially controlled cells inside the 3D neuronal network. A combination of the well-established MEA design and a 3D printed polymer scaffold structure on top of it is the device of choice. Electrodeposition of gold is used to create the electrodes which reach into the several cage planes.
CONTACT:
Prof. Dr. Andreas Offenhäusser
Tel.: +49-2461-61-2330
e-mail: a.offenhaeusser@fz-juelich.de